Coffee Water – What You Need to Know
Did you know that your coffee consists of over 90% water? Yet, water quality is often overlooked when brewing coffee. Hard water can damage your machine and, if poorly composed, negatively affect the flavor of your coffee. Here are the liquid facts:
Coffee Water – What You Need to Know
- What is Water Hardness?
- How Hard is Your Tap Water?
- How Does pH Value Affect Coffee?
- Our Tips for Better Coffee Water
- Magnesium or Calcium – Which One Is Better for Your Coffee?
- How a Tabletop Water Filter Works
- Bottled Mineral Water from the Supermarket
- Third Wave Water
- Reverse Osmosis (RO) Systems and Remineralization
- TDS Meters
- Conclusion
Although minerals make up only a small portion of our drinking water, they have a major impact on taste and extraction. When it comes to coffee preparation, dissolved minerals like calcium and magnesium (total hardness), as well as hydrogen carbonate (carbonate hardness), are especially important. The water’s pH level also plays a role. Depending on local geology and soil conditions, water hardness and pH can vary significantly.
In a Hurry? Here’s Your 1-Minute Summary:
What is Water Hardness?
Total water hardness refers to the concentration of calcium and magnesium ions. We distinguish between total hardness and carbonate hardness, the latter indicating the portion of hardness caused by hydrogen carbonate compounds (HCO₃⁻). Both values influence extraction and flavor in different ways. High total hardness allows for more compounds to be extracted from the coffee. In terms of taste, calcium tends to emphasize sweetness, while magnesium highlights bitter notes.
Rule of thumb: The higher the ion concentration, the higher the total hardness of the water.
Carbonate hardness is crucial for the water’s buffering capacity (i.e., how much the pH level shifts when in contact with coffee components). Too little carbonate hardness results in an unstable pH, which can damage equipment over time and overly emphasize the coffee’s acidity. Too much carbonate hardness neutralizes coffee acids, making the flavor taste “flat.” When water with high carbonate hardness is heated, the hydrogen carbonate reacts with calcium or magnesium ions – forming limescale that deposits as a hard, gray residue. These limescale deposits not only reduce your machine’s efficiency but can also shorten its lifespan. This is especially problematic for espresso machines and fully automatic machines, where limescale can build up in pipes, boilers, and the brew group – potentially resulting in costly repairs. High limescale content can also distort your coffee’s flavor balance, muting subtle acidity and sweetness.
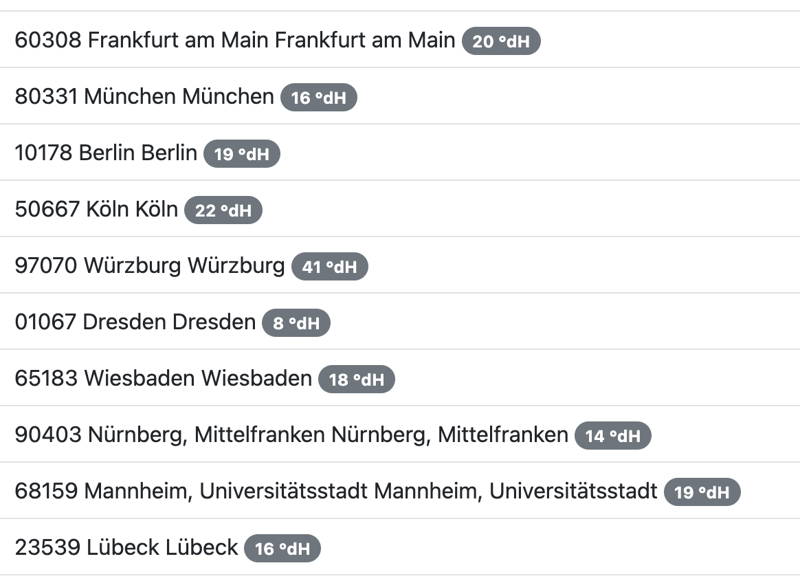
How Hard is Your Tap Water?
Most households in Germany have tap water with carbonate and total hardness levels that are too high. We’ve compiled a chart of water hardness in major cities.
You can check your local total water hardness using websites like www.waterhardness.net by entering your postal code. In Germany, hardness is measured in “degrees of German hardness” (°dH), which primarily reflects calcium and magnesium ion content. Don’t be surprised! Some guides use a different system like ppm CaCO₃ (parts per million of calcium carbonate). The conversion is: 1 °dH = 17.848 ppm CaCO₃. To make things easier, we’ve converted the recommended values for you in this article.
How Does pH Value Affect Coffee?
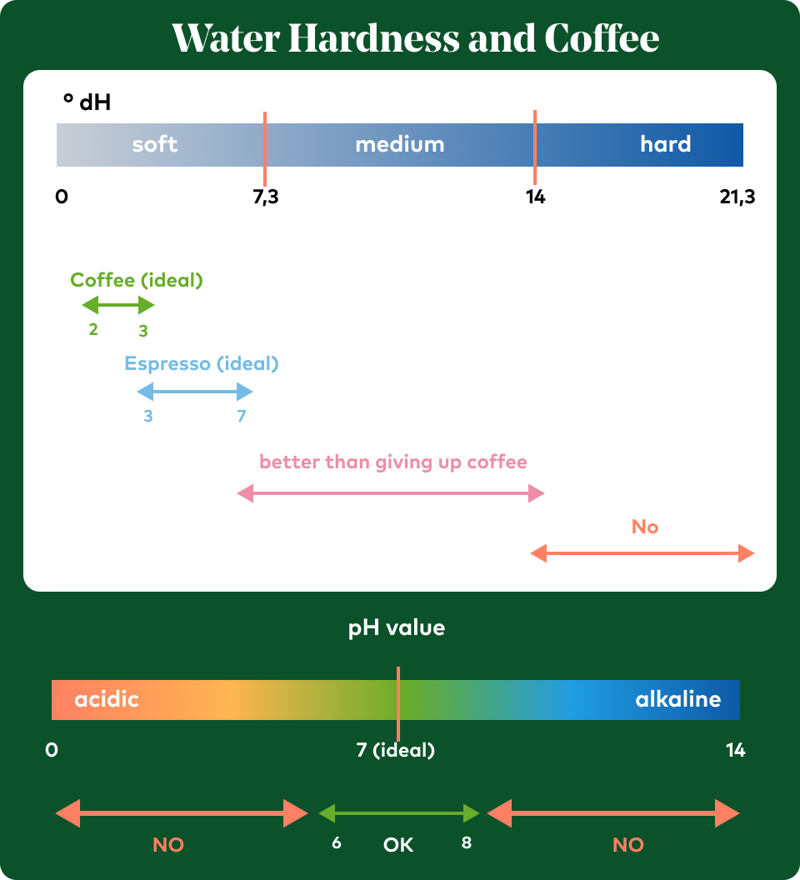
By now, you’ve probably noticed how important the combination of pH and carbonate hardness is for good coffee water. While the pH value doesn’t directly determine hardness, it’s crucial for balancing the acid-base interaction and buffering capacity. It affects how strongly carbonate hardness acts as a buffer against acids. A pH that’s too high, like too much carbonate hardness, can neutralize the coffee’s delicate acidity, resulting in flat flavor. Maxwell Colonna-Dashwood and Christopher H. Hendon recommend using slightly acidic to neutral water for coffee brewing (depending on composition), which corresponds to a pH of 6.5-7.5.
| Term | Refers to… | Impact on Coffee |
|---|---|---|
| Total hardness | Total Calcium + Magnesium | Extraction, taste |
| Carbonate hardness | Portion of it as hydrogen carbonate (HCO₃⁻) | pH stability, acid balance, limescale |
| pH value | Measure of acidity | Affects flavor and buffering behavior |
Sounds Complex? It Is – But We’ve Got You!

Our Tips for Better Coffee Water
According to Water for Coffee, the optimal hardness level for coffee is approximately 3-7 °dH for espresso and 2-3 °dH for filter coffee. Machine manufacturers recommend a carbonate hardness of below 4 °dH to prevent limescale buildup. For high-end espresso machines, many manufacturers now even recommend a carbonate hardness of only 0-3 °dH to largely eliminate scale formation. However, this does affect flavor. Extremely soft or fully demineralized water, with hardness levels below 1-2 °dH, can lead to unbalanced extraction: acidity becomes overly pronounced, while body and sweetness are lacking. On the other hand, higher carbonate hardness can cause the water to become more alkaline, which neutralizes the coffee’s delicate fruit acids. Arabica coffees, in particular, lose vibrancy and complexity as a result. That’s why many home baristas now use reverse osmosis systems or distilled water combined with targeted remineralization. Based on the guidelines from the Specialty Coffee Association (SCA) and Water for Coffee, the following limits are recommended:
| Parameter | Recommended Ranges (Water for Coffee) | Converted to °dH |
|---|---|---|
| Calcium hardness | 10–50 mg/l Ca²⁺ | ca. 1,4–7 °dH (calcium portion of the total hardness only; 1 °dH = 7,14 mg/l Ca²⁺) |
| Magnesium hardness | 5–30 mg/l Mg²⁺ | ca. 1,2–6,9 °dH (magnesium portion only; 1 °dH = 4,34 mg/l Mg²⁺) |
| Carbonate hardness | 40–70 ppm (as CaCO₃) | ca. 2,2–4 °dH (1 °dH = 17,848 mg/l CaCO₃) |
| TDS (Total Dissolved Solids) | 75–250 ppm | No direct conversion possible, since TDS represents the total of all dissolved substances – not just hardness-forming minerals |
| pH value | 6,5–7,5 | - |
What Do the Abbreviations Mean? A Glossary
| Term | Explanation |
|---|---|
| Ca²⁺ (Calcium ions) | Positively charged calcium ions. Important for extraction and part of total hardness. However, too much can cause scaling in machines. |
| Mg²⁺ (Magnesium ions) | Positively charged magnesium ions. Also important for extraction, often more flavor-enhancing than calcium.. |
| CaCO₃ (Calcium carbonate) | Reference value used to express water hardness (especially carbonate hardness). Indicated in mg/l or ppm. |
| TDS (Total Dissolved Solids) | Total of all dissolved substances in the water (e.g., minerals, salts, metals). Does not indicate hardness alone. |
Magnesium or Calcium – Which One Is Better for Your Coffee?
It’s not just the quantity of minerals but also their type. According to Water for Coffee, magnesium enhances flavor more than calcium, as it binds more strongly to aromatic compounds. Calcium mainly contributes to mouthfeel and body but causes more scaling. Ideal coffee water contains a balanced mix of both – for full flavor and healthy machines.
These exact specifications are important if you want to dive deeper into remineralization. But there’s an easier way!
This graphic gives you a quick overview of how you can influence your coffee’s flavor with less effort:
The simpler and more cost-effective alternative to reverse osmosis systems, built-in filters, and targeted remineralization is a tabletop water filter. With Brita Professional, you need to change the filter regularly. However, with moderate use, the ongoing costs remain relatively low compared to the alternatives mentioned – especially for tea and coffee drinkers who boil their water before consumption. The filtration also results in a nearly limescale-free kettle and a relatively pure coffee flavor.
A tabletop water filter consists of four different filtration stages. In the pre-filtration, coarse particles are removed. In the next step, a so-called ion exchanger replaces scale-forming ions with sodium ions, reducing the carbonate hardness. The activated carbon filter removes unpleasant tastes and odors, such as chlorine. Finally, the fine filtration traps even the smallest particles and heavy metals. Depending on the cartridge and the source water, certain amounts of calcium and magnesium may also be reduced by the filter and may need to be re-added afterward, as their absence can affect flavor. A downside of ion exchange filters is that they often can only be regenerated by the manufacturer once they are saturated. For private users, this is usually not an option – so the only solution is to replace the filter regularly. Additionally, with very hard tap water, it may be necessary to filter the water more than once, since a tabletop filter typically only reduces the hardness by about half per cycle. Depending on your region, this might not be sufficient to reach the optimal hardness level.
Contrary to popular belief, tabletop filters are not designed to remove germs from water. In fact, the containers can become prone to bacterial contamination. Although antibacterial substances are added to the filters, they wash out over time, which makes it all the more important to replace filter cartridges regularly.
Soft to Moderately Hard Mineral Water
There are, of course, simple and affordable alternatives to a tabletop filter. The simplest solution is to find the perfect mineral water for you and your taste. A trip to the supermarket might be all it takes to solve the mystery. As mentioned above, the key factor is the total hardness, which is primarily determined by the magnesium and calcium content. Make sure that the difference between magnesium and calcium is not too large. Let’s take a look at a few still mineral waters in terms of their total hardness as well as magnesium and calcium content:
- Volvic (3,4 °dH) / Magnesium: 8 mg/l; Calcium: 12 mg/l
- Spreequell (4-5 °dH) / Magnesium: 3,3 mg/l; Calcium: 17 mg/l
- Fiji Water (5,98 °dH) / Magnesium: 13 mg/l; Calcium: 17 mg/l
- Evian (17,21 °dH) / Magnesium: 26 mg/l; Calcium: 80 mg/l
- Adelholzener (17,44 °dH) / Magnesium: 30,4 mg/l; Calcium: 92,8 mg/l
- Vittel (17,79 °dH) / Magnesium: 20 mg/l; Calcium: 94 mg/l
- Gerolsteiner (30,93 °dH) / Magnesium: 44 mg/l; Calcium: 125 mg/l
As you can see, based on hardness alone, only three of these mineral waters are suitable for brewing a good cup of coffee. Aside from Spreequell, Volvic, and the relatively hard-to-find and premium Fiji Water, all others are either too soft or too hard. Volvic and Fiji Water have the added advantage that the difference between their magnesium and calcium content is not too large. The former has a higher extraction potential than the latter. Still, neither offers perfect extraction. However, they can be a simple way to improve your coffee water and serve as a great introduction to this complex topic.
Even though mineral waters like Volvic, Spreequell, or Fiji can work well as a temporary solution or a starting point, many coffee enthusiasts today prefer to mix their own water with a more precisely balanced mineral profile – for example, using products like Third Wave Water or remineralization kits for reverse osmosis systems.

Current Coffee Water Trends 2025 Update
Third Wave Water
If you don’t want to keep buying mineral water for your coffee brewing, you can try another alternative to a tabletop filter: Third Wave Water. This U.S.-based company has made it its mission to produce the perfect water for coffee. However, making it is a bit more involved than simply buying mineral water or filtering your tap water.
Third Wave Water is especially popular in the specialty coffee scene because it provides a standardized, reproducible water profile – precisely tailored to the SCA (Specialty Coffee Association) guidelines. The company offers three different product types: one for light to medium roast filter coffee, one for dark roasts, and one for espresso preparation. Let’s say you want to brew a light to medium roast filter coffee: the Third Wave Water Classic Profile pack contains twelve sachets. Each sachet holds a precise amount of magnesium sulfate, calcium citrate, and potassium bicarbonate – exactly what you need for your filter coffee brew. You mix one sachet into 3.785 liters (1 gallon) of distilled water. Stir or swirl gently, and you’ll have perfectly balanced coffee water, made to SCA specifications. The water will now contain the ideal levels of magnesium and calcium, and the correct hardness.
Reverse Osmosis (RO) Systems and Remineralization
Reverse osmosis systems are becoming increasingly popular – especially among espresso drinkers with high-end machines. When combined with remineralization cartridges or salts, they allow you to produce water that is both free of scale and optimized for flavor. However, the initial investment typically starts in the low four-digit range, making them not always affordable.
Reverse osmosis systems (also called RO systems) are now considered the gold standard for achieving optimal water quality for high-quality coffee.
In these systems, tap water is forced through a semi-permeable membrane that removes up to 99% of all dissolved substances (e.g., limescale, chlorine, heavy metals, and minerals). The result is nearly pure H₂O – free from unwanted flavors or scale. Many modern devices now offer smart app controls or cartridges with customizable mineral profiles. It’s also common to find systems that combine pre-filters, activated carbon filters, and remineralization stages.
Since water that’s too “empty” isn’t ideal for coffee brewing, it is deliberately remineralized afterward. Some coffee enthusiasts also start with distilled water to build their own mineral profile from scratch.
There are two common methods for this:
- Remineralization cartridges that add measured amounts of magnesium, calcium, and carbonates back into the water.
- Powdered solutions like the previously mentioned Third Wave Water, which are mixed with distilled or RO water – ideal for creating precise water profiles.
The advantage: You can fine-tune your coffee water to match your brewing method, roast level, and taste preferences – while maintaining consistent water quality and eliminating any risk of scale buildup in your machine.
TDS Meters
If you want to optimize your coffee water, you need to understand it – and measure it. That’s why many home baristas today use TDS meters (Total Dissolved Solids). These devices display the amount of dissolved substances in water in ppm (parts per million) – a practical reference point for assessing water quality.
A TDS value between 100–150 ppm is considered ideal for most brewing methods. If your value is too high or too low, you can adjust it using filters, dilution, or targeted remineralization. TDS gives a general indicator of total mineral content – but not the specific composition (e.g., the ratio of magnesium to calcium). For fine-tuning, additional analysis or dedicated apps are helpful.
With apps like the Barista Hustle Water Calculator or the Aquacode Water Calculator, you can digitally plan and optimize your water profile. These tools help calculate the ideal balance of calcium, magnesium, and carbonate hardness depending on your brewing method (filter, espresso, cold brew) and coffee bean.
The result? More transparency, more consistency – and more flavor.
More and more coffee enthusiasts are now designing their water based on bean type – with fruity coffees benefiting from more magnesium, and bolder roasts from a more balanced calcium-magnesium ratio.
Conclusion
Before you consider purchasing a filter, find out the hardness of your water first and whether a water filter is necessary. The easiest way is to identify your local water supplier at wasserversorger.de. On this site, you can find the relevant water quality values for your area. Alternatively, you can order free water hardness test strips from the company Brita. These test strips are also available at pharmacies or drugstores. Within seconds, you can determine your water’s hardness level with these strips.
You know your hardness level? Now all that’s left is to use our guide to decide which method suits you best.
May your coffee water always have the perfect hardness!